A practical example of Activator use: The Iodine for thyroid examination.
Iodine isotopes are widely used for thyroid examination.
The element is initially available in the most appropriate chemical compound
(for instance NaI) made with natural Iodine (stable isotope 127
I).
Shortly before the administration, the compound is introduced in the Activator
driven by a small proton accelerator (23 MeV, 1 mA) and activated, during
a time of the order of one half-life (25 min), with the help of the reaction:
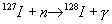 , which transforms natural Iodine into the tracing element 128
I with beta decay.
, which transforms natural Iodine into the tracing element 128
I with beta decay.
There is no chemical “preparation” between activation and examination.
This very simple procedure is becoming practical with the Activator because
of the higher efficiency of neutron capture, which produces the required
Iodine strength (~1 GBq (1)) starting form a tiny initial amount of natural
Iodine (~ 1 gram), and using a conventional accelerator like those already
in wide use in hospitals for other applications like PET.
This method makes practical the use of 128I as a tracing element
for thyroid diagnostics with a much shorter half-life (25 min) than the
other currently used Iodine isotopes (131I –8 days- and
123 I -13.2 hours-) and the corresponding important advantage of a
much smaller dose to patients, extending the applicability to cases of young
patients or pregnant women.
Index
Back